Research
We build custom microscopes using tools from the physics laboratory to realize new biological measurements. A common theme across our work is the use of time and frequency-domain optics to drive new imaging approaches.
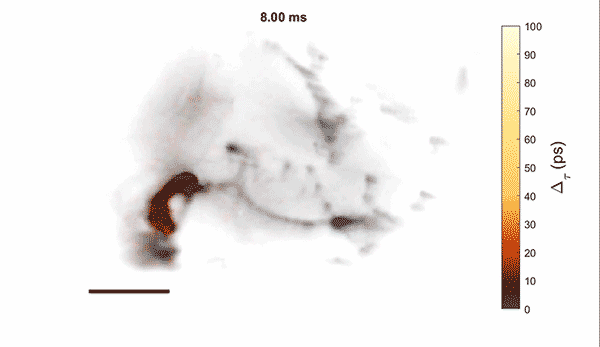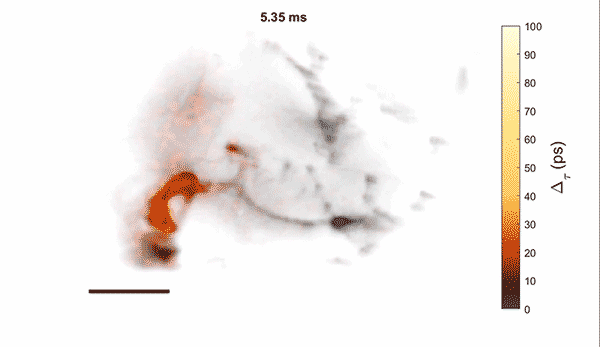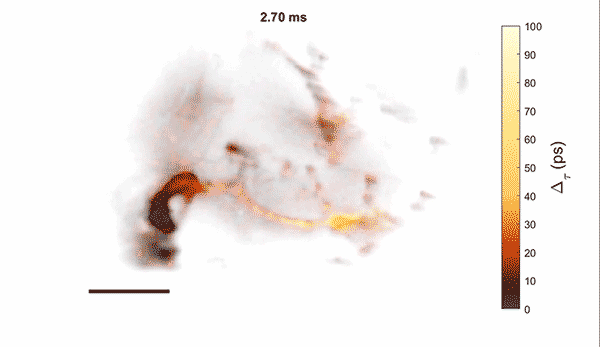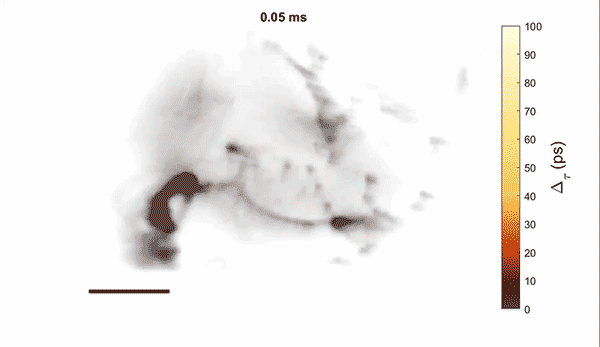
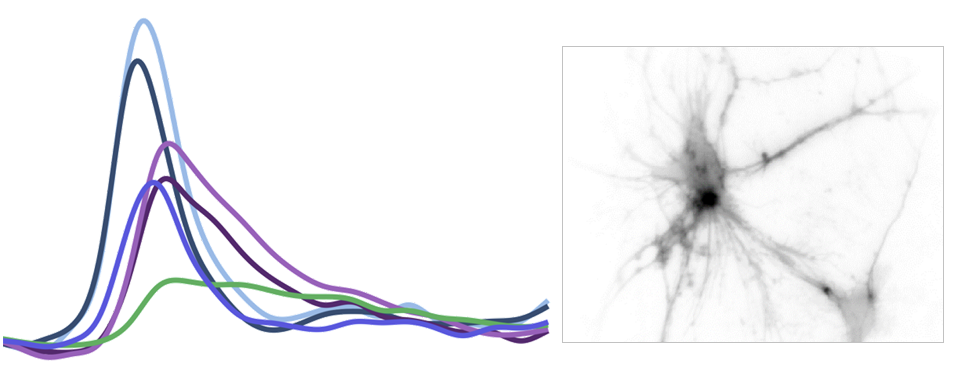
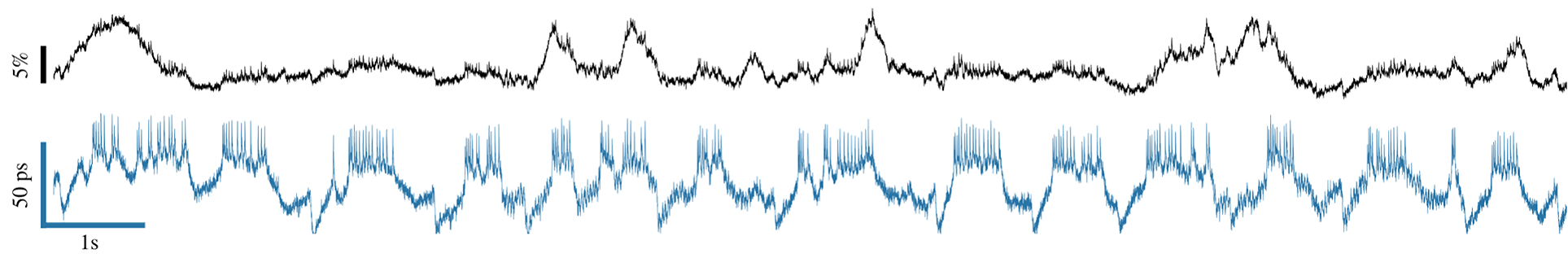
Measurement of cellular signaling: Most scientific cameras are exquisitely sensitive but process images slowly, meaning that faster dynamics cannot be captured without sacrificing performance. Electro-optic fluorescence lifetime microscopy (EO-FLIM) uses polarization modulators to capture nanosecond lifetime on these standard cameras. We have applied EO-FLIM in several contexts ranging from single-molecule microscopy to kilohertz rate recording of neuron membrane potential in vivo. Lifetime readout improves measurement stability and rejection of sample motion and noise artifacts. It has also proven capable of capturing action potential propagation and sub-threshold voltage signals throughout neuron structures. We are developing next-generation EO-FLIM microscopes to improve measurements of functional signals and extend them to 3D tissue environments. These efforts combine instrument development with the latest genetically-encoded fluorescent indicators to address fundamental questions in neuroscience.
Imaging at physical limits: Most microscopes are ultimately limited by achievable signal-to-noise ratio and sample damage. In many cases, the imaging laser itself causes damage to the sample by heating, bleaching fluorophores, or inducing chemical reactions. Live samples can also introduce their own noise from motion and hemodynamic effects, and non-ideal instruments can add electronic noise. We are broadly interested in techniques to overcome experimental noise sources and damage limits for optical microscopy. Nanosecond optics provide an exciting tool to realize noise-robust imaging protocols and to achieve imaging performance near physical limits imposed by shot noise.